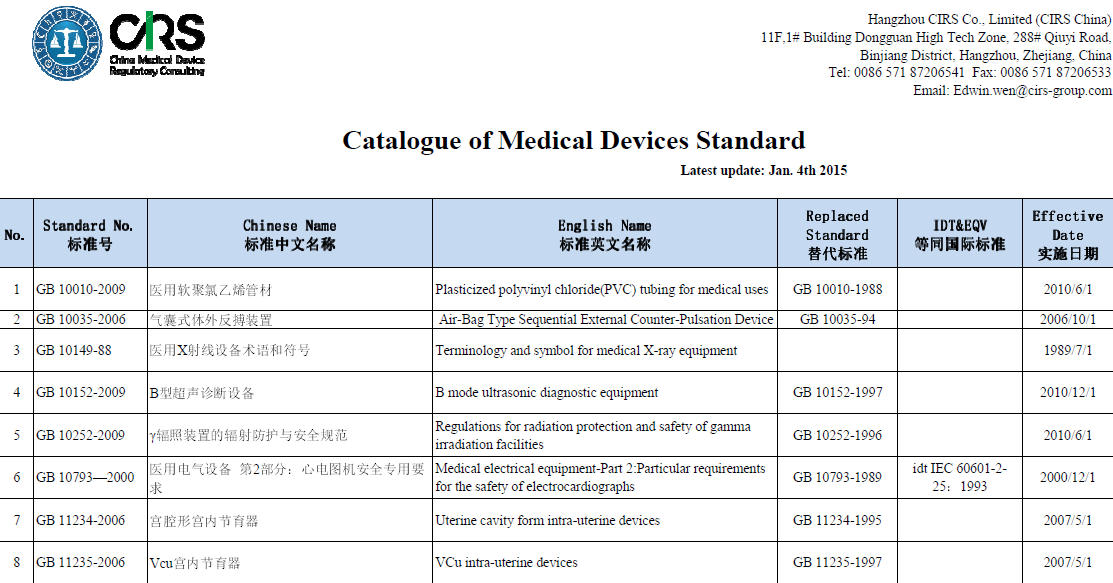
The product technical requirement for medical device shall be compiled according to the China related national standard of medical product. It is the most important document for product safety and effectiveness evaluating and the appendix of the medical device registration certificate.
CIRS offers normally used national & professional standards, and technical requirements for your convenience. Typically, most of standards we provide are in Chinese version currently, the English ones are under translation now and will be availablle for you as soon as possible. The Catalogue of Medical Devices Standard can be found here, or you can contact Mr. Edwin Wen (Edwin.wen@cirs-group.com) to search the relevant product standard.