General Information
In 2013, after China officially joined the International Medical Device Regulators Forum (IMDRF), National Medical Products Administration (NMPA, former CFDA) issued the “Regulations on the Legislative Procedures of the State Food and Drug Administration” for the purpose of integrating with the international mainstream and better catching up with the development of the medical device industry. This regulation is issued to standardize the legislative process, ensure the quality of legislation, and improve the efficiency of legislation. Based on this rule,NMPA issued a new “Regulation on the Supervision and Administration of Medical Devices” in 2014 and revised it in 2017.
To guarantee the safety and effectiveness of medical devices, any foreign enterprise engaged in the manufacture, preparation, propagation, compounding, or processing of a medical device imported into theChina and wants to record or register their products in China, must conduct the record keeping or registration in accordance with the<Regulations for the Supervision and Administration of Medical Device>issued by NMPA.
The “market approval” released by your national government is necessary for the imported medical device, which is a legal proof/certificate/license demonstrates that your product is approved to be sold as a medical device in the market of your country.
For Class Ⅰ medical device, you shall implement the record keeping; while for ClassⅡ&Ⅲmedical device, you shall implement the registration. Either the record keeping or the registration is in the charge of NMPA(orginal CFDA).The general required preparation dossiers would include:security risk evaluation report, product standard (technical requirement), product testing, clinical evaluation report, manual and label, quality management system etc
You can see below to find detailed preparation dossiers for record/registration procedure.
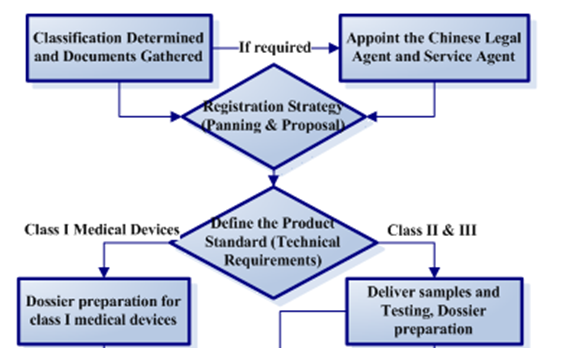
For more procedure details, please clickhere.
For more informations, please contact Edwin Wen for further assistant:
Email address:Edwin.wen@cirs-group.com
CIRS services
Medical Device Registration
- Regulatory Compliance Assessment and Registration Consulting
- Product Classification attributes Defined and Registration Proposal Cofirmed
- Registration(Recording) for Medical Device and In Vitro Diagnostic (IVD)
- Registration Certificate Update and Renewal for Medical Devices and IVD
- Coordinate the Registration Inspection and Commissioned Inspection
- Compliation and Submission of Technical Construction File
Medical Device Clinical Evaluation
- Clinical Trials for Medical Devices and IVD
- Pre-clinical Investigation of Medical Device
- Clinial Trial Protocal Design for Medical Device
- Clinical Trials Auditing and Quality Assurance
- Data Mnagement and Statistical Analysis
- Draft Clinical Trial Report and Clinical Evaluation Report
- Medical Device Clinical Monitoring
Medical Device QMS Service
- Initial Analysis and Confirmation when QMS Establishing
- QMS Designing and Consulting
- QMS Establishment and Operation Consultation
- Training of QMS and Self-inspection
- QMS on-site audit and correction consultation
- ISO 13485 QMS establishement and Consultation







