CFDA has released the 2015 Food And Drug Regulatory Statistical Annual Report, which announced the objective data about health food, drug, medical device and cosmetic. The data involved with manufacture, distribution, registration, and so on.
In this respect, CIRS would like to share the medical device regulatory data from 2012 to 2015 with people who may concern.
In this respect, CIRS would like to share the medical device regulatory data from 2012 to 2015 with people who may concern.
1. Medical Device Manufacturer & Distributor
Up to the end of Nov 2015, there were total 14151 medical device manufacturers in China, in which 5080 are Class I manufacturers, 9517 are Class II manufacturers, and 2614 are Class III manufacturers.
Up to the end of Nov 2015, there were sum up to 186269 medical device distributors in China, not including the distributors which sell Class I medical device. The number of Class II distributors is 125197, and Class III distributors is 121984.
Comparing the history data (2012~2015), see the following tables:

From the above two tables, we can find that the total number of manufacturers and distributions of 2015 have decreased compared to 2014. But, the number of Class I manufacturers has increased. Reason for this phenomenon may include the GMP and GSP inspection.
Up to the end of Nov 2015, there were sum up to 186269 medical device distributors in China, not including the distributors which sell Class I medical device. The number of Class II distributors is 125197, and Class III distributors is 121984.
Comparing the history data (2012~2015), see the following tables:

From the above two tables, we can find that the total number of manufacturers and distributions of 2015 have decreased compared to 2014. But, the number of Class I manufacturers has increased. Reason for this phenomenon may include the GMP and GSP inspection.
2. Medical Device Registration
Up to the end of 2015, CFDA has recorded 11055 Class I medical device and approved 11628 Class II medical device, 1661 Class III medical device, in which, the number of medical devices which from Hong Kong, Macao and Taiwan is 121, and imported medical devices is 1559.
The detailed data, see following table:
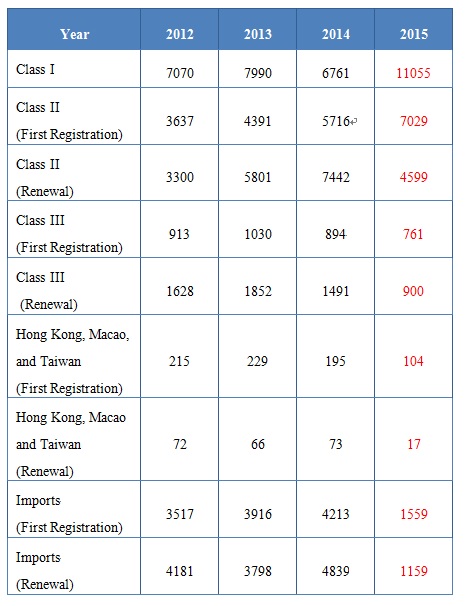
The detailed data, see following table:

From the above table, we can find that the number of Class I medical device has increased sharply, as well as Class II medical device of first registration.
If you want to know more information about what was happening in China of 2015 and what is going to happen in China of 2016, you can take part in the free webinar: China Medical Device Regulatory YearReport-2015.
If you want to know more information about what was happening in China of 2015 and what is going to happen in China of 2016, you can take part in the free webinar: China Medical Device Regulatory YearReport-2015.

