In order to strengthen the supervision and management of medical device distribution, standardize and guide the GSP’s on-site inspection, CFDA has formulated the Guideline for Medical Device Good Supply Practice On-site Inspection (hereinafter referred to as Guideline), and issued on Oct.15th , 2015.
The Guideline applies to on-site inspection for medical device wholesale/retail distributors of Class II and Class III, as well as to all kinds of supervision and inspection for medical device distributors.
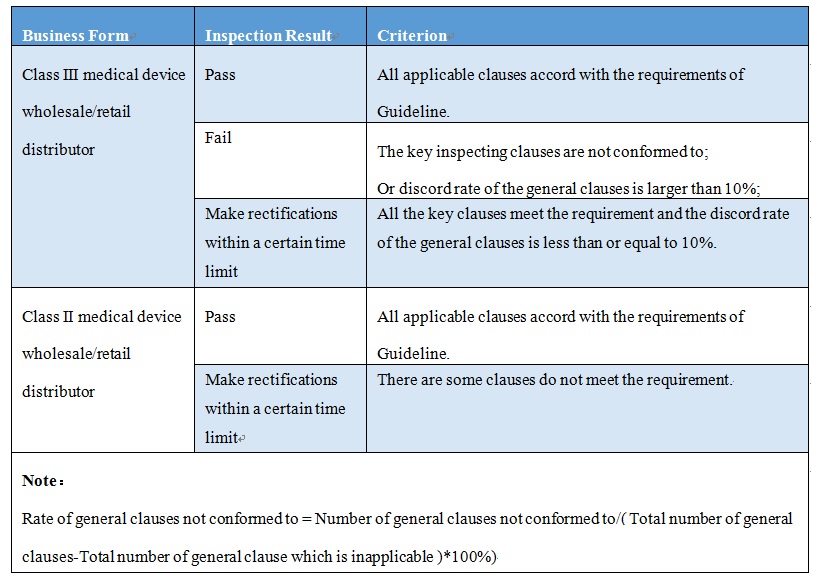
The Guidance regulates departments' GSP on-site inspection to medical device distributors and evaluation of the inspection results. When conduct the on-site inspection, the supervisors and inspectors shall check the condition of medical device distributor according to inspection clauses and its corresponding key inspection content which are detailed in the Guideline. If there are some inspecting clauses of Guideline do not apply to your distributor, you can determine the reasonable lack clauses according to business mode, business scope, and line of business, etc., then submit the written document to inspecting group to conduct confirmatory by inspecting group.
Inspection result:
The Guideline applies to on-site inspection for medical device wholesale/retail distributors of Class II and Class III, as well as to all kinds of supervision and inspection for medical device distributors.
The Guidance regulates departments' GSP on-site inspection to medical device distributors and evaluation of the inspection results. When conduct the on-site inspection, the supervisors and inspectors shall check the condition of medical device distributor according to inspection clauses and its corresponding key inspection content which are detailed in the Guideline. If there are some inspecting clauses of Guideline do not apply to your distributor, you can determine the reasonable lack clauses according to business mode, business scope, and line of business, etc., then submit the written document to inspecting group to conduct confirmatory by inspecting group.
Inspection result:

If the result of inspection is rectifications within a time limit, you shall complete the rectification and reform, and submit the one time rectification report to original inspection department within 30 days after on-site inspection. The department would organize the supervisor and inspector to conduct review, if all the rectification clauses accord with the requirements, you can pass the inspection. If you cannot complete the rectification in time, you will fail to pass the inspection.
During inspection, if inspectors find other actions which violate the relevant provision of “Regulations for the Supervision and Administration of Medical Devices” and “Administrative Measures for the Supervision of Distribution of Medical Devices”, inspector can handle them according to the corresponding laws and regulations.
When the inspection ends, inspection group shall fill in the Medical Device Good Supply Practice On-site Inspection Form and Medical Device Good Supply Practice On-site Inspection Report.
If you need the English version of Guideline for Medical Device Good Supply Practice On-site Inspection, please contact us.
Ms. Windy Jin,
Medical Device Legislation Compliance Deportment, CIRS China;
11F Dongguan Building, 288 Qiuyi Road, Binjiang District, Hangzhou, China, 310020
Email: Windy@cirs-group.com
During inspection, if inspectors find other actions which violate the relevant provision of “Regulations for the Supervision and Administration of Medical Devices” and “Administrative Measures for the Supervision of Distribution of Medical Devices”, inspector can handle them according to the corresponding laws and regulations.
When the inspection ends, inspection group shall fill in the Medical Device Good Supply Practice On-site Inspection Form and Medical Device Good Supply Practice On-site Inspection Report.
If you need the English version of Guideline for Medical Device Good Supply Practice On-site Inspection, please contact us.
Ms. Windy Jin,
Medical Device Legislation Compliance Deportment, CIRS China;
11F Dongguan Building, 288 Qiuyi Road, Binjiang District, Hangzhou, China, 310020
Email: Windy@cirs-group.com

