The guideline applies for application and handle of registration alteration of imported medical device.
2. Item information
- Project name: Examination and approval for imported medical device registration;
- Subitem name: Examination and approval for imported medical device registration alteration;
The article 14 of Regulations for the Supervision and Administration of Medical Devices has stated that if the design, raw materials, production process, application scope, usage methods, etc. of the medical devices have been changed substantially, such may affect the safety and effectiveness of medical device, the applicant shall apply for changing the registration to the original registration department. If the change is not substantial and does not affect the safety and effectiveness of medical device, just submit the change to the original registration department for record.
4. Accepting institution
Center for Medical Device Evaluation.CFDA (hereafter refers as MDE)
5. Decisive institution
China Food and Drug Administration (hereafter refers as CFDA)
6. Application condition
Applicant shall be oversea manufacturer, and the medical device has obtained the medical device registration in China.
7. Application documents list
- Application form;
- Supporting document;
- A statement about change of medical device issued by manufacturer;
- The copy of original registration certificates and its attachments of medical devices;
- Comparison table and explanation of medical device change;
- Risk management report related to medical device change;
- The document related to the influence of change part on safety and effectiveness;
- Registration inspection report related to change part;
- Declaration of conformity.
9. Fee standards
Medical device of Class II: RMB 42,000;
Medical device of Class III: RMB 50,400
10. Approval result
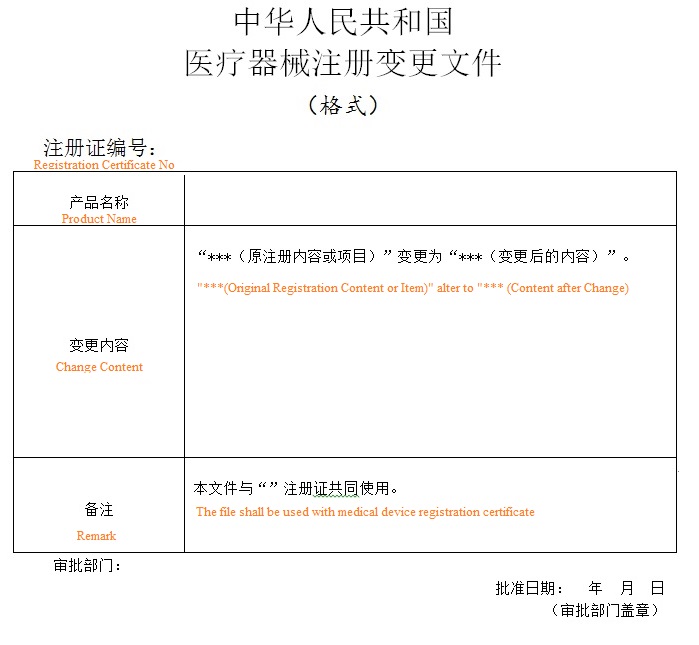
http://www.cirs-md.com/news_and_events/Tips_CFDA_Registration.html

