
China Food and Drug Administration (hereinafter refers to as CFDA) has issued the Medical Device Registration Report in 2015 on Apr.1st, 2016.
In 2015, CFDA took it as its own duty to maintain and promote public health, thoroughly implemented Regulations for the Supervision and Administration of Medical Device, and promoted approval system reform for medical device, standardized and guided national medical device registration to enhance the quality and efficiency of medical device registration approval.
1. Working condition of medical device registration
According to Regulations for the Supervision and Administration of Medical Device, CFDA is in charge of medical device registration, technical review and administration approval for domestic class III, imported class II and class III. The provincial FDA is in charge of domestic class II.
1.1 Improve regulation system of medical device registration management
CFDA has formulated and issued 16 regulations and normative documents such as Medical Device Classification Rules, Naming Rule of Medical Device Generic Name, and Guideline of Medical Device Clinical Trial Evaluation, etc. CFDA improved the regulation system of medical device registration management and standardized medical device technical review, registration verification and other related work to provide good legal foundation for provincial FDA.1.2 Promote approval system reform for medical device
In 2015, CFDA has implemented programs of approval system reform for medical device to further strengthen pre-job training and continuing education training for reviewer and improve comprehensive quality of reviewer.CFDA has issued 28 national standards and 90 industrial standards, formulated Reform of Medical Device Classification Management, established technical committee of medical device classification, and stated the revised work of Medical Device Classification Catalogue. CFDA continued to promote innovative medical device development in according with Special Approval Process for Innovative Medical Device (Trial).
1.3 Conduct ability assessment of review and approval for provincial FDA
CFDA has issued Guidance on Strengthening Technical Review Ability of Provincial FDA, formulated Ability Assessment Program of Review and Approval for Provincial FDA, and conducted ability assessment to improve the level and quality of medical device registration management.1.4 Strengthen basic work of registration
CFDA continued to promote revised work for Guideline of technical review of medical device registration, and has issued 125 Guidelines of technical review of registration and 2 guidelines of instruction compilation.CFDA has enabled the Class I Record System of Medical device Registration Management, to standardize and guide Class I record on Sep 10th, 2015.
CFDA has enabled the new medical device registration management system to standardize electronic application requirements, and improve information level and work efficiency of registration review on Dec 20th 2015.
2. Acceptance situation of medical device registration application
CFDA has accepted 9396 applications of medical device registration, in which: 2402 first registration applications, 5105 renew registration applications, and 1889 applications of licensing item change. A 6.7% reduction compared to the application number of 2014
2.1 Overall situation
By 2015, there are 3921 registration applications of domestic Class III which include in vitro diagnostic reagents, and 5475 of imported medical device.- Distinguished by registration catalogue, medical device application number is 5509, and in vitro diagnostic reagent is 3887.
- Distinguished by registration form, first registration application number is 2402, renew application is 5105, and licensing item change application is 1889.

Figure 1. Scatter gram of Registration Forms
2.2 Itemization situation
- Domestic Medical Device of Class III
Distinguished by registration form, first registration application number is 1562, account for 40%; renew application is 1635, 42%; and licensing item change application is 724, 18%.
- Imported Medical Device of Class II
Distinguished by registration form, first registration application number is 499, account for 17%; renew application is 1895, 65%; and licensing item change application is 630, 20%.
- Imported Medical Device of Class III
Distinguished by registration form, first registration application number is 341, account for 14%; renew application is 1575, 64%; and licensing item change application is 535, 22%.
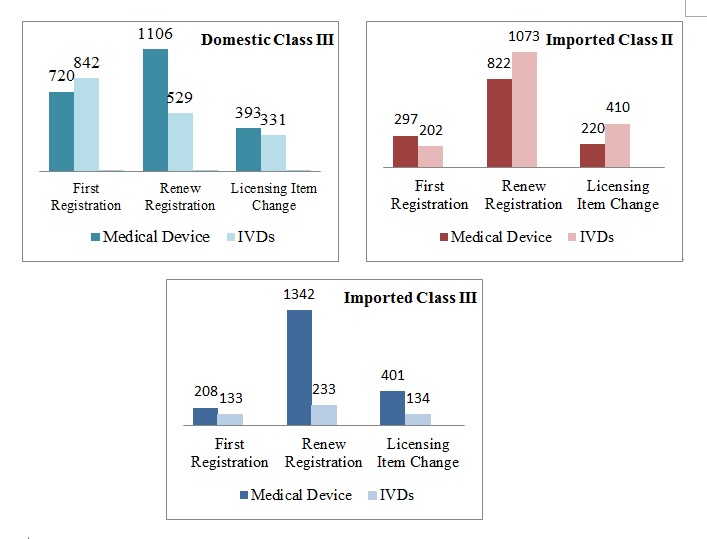

Figure 2. Scatter gram of Registration Forms of Domestic Class III, Imported Class II and Class III.
In summary, the number of renew application is larger than the first registration application, except the IVDs of domestic class III. And whether domestic or imported, the numbers of licensing item change application are same, are 20% or so.3. Review and Approval Situation
3.1 Overall situation
By 2015, CFDA has approved 7530 applications of medical device registration, in which the number of first registration applications is 2707, the renew registration applications are 4072, the applications of licensing item change are 751. A slight reduction compared to the approval number of 2014 and 2013.There 1297 registration application were not be approved by CFDA in 2015.

Figure 3. Scatter gram of registration data (2013~2015)
There are 2730 registration applications of domestic class III are approved, as well as 4800 application of imported class II and class III, which include IVDs.
Figure 4. Scatter gram of registration data of domestic and imported medical device in 2015
Distinguished by registration form, the proportional distribution as following:

Figure 5. Scatter gram of registration form.
3.2 Itemization situation
- Domestic Class III
For IVDs, CFDA has approved 514 first registration applications, 479 renew registration applications, and 243 applications of licensing item change.
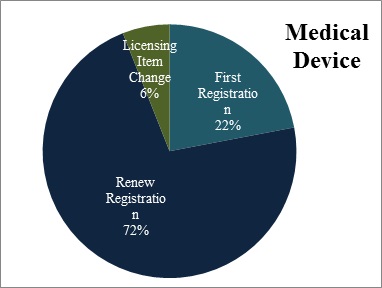

Figure 6 Scatter gram of registration form of Domestic IVDs and Medical Device
- Imported Medical device (including IVDs)
For imported IVDs, CFDA has approved 326 first registration applications, 879 renew registration applications, and 266 applications of licensing item change.

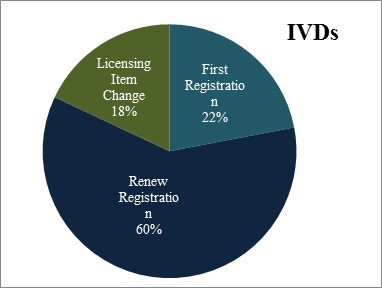
Figure 7. Scatter gram of registration form of Imported IVDs and Medical Device
- Catalogue analysis of approved medical device
IVDs of domestic class III make up 45% of domestic product, and imported IVDs make up 31% of imported product.
By 2015, except IVDs, domestic medical device involve with 26 subdirectories of Medical Device Classification Catalogue.
The top five products of registration quantity are: Biomedical polymer material and product, implant and artificial organ, injection and puncture instrument, medical optical instrument and endoscope device, and insert device.
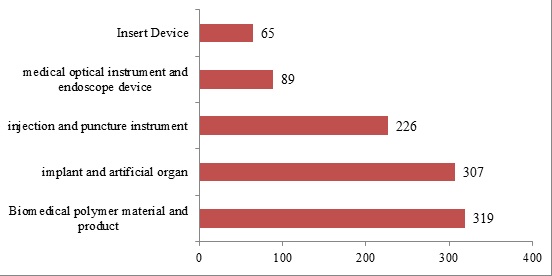
Figure 8. Domestic Medical Device Ranking
By 2015, except IVDs, imported medical device involve with 41 subdirectories of Medical Device Classification Catalogue.The top five products of registration quantity are: medical optical instrument and endoscope device, implant and artificial organ, dental material, medical electronic equipment, biomedical polymer material and product,

Figure 9. Imported medical device ranking
- Source Analysis of imported medical device

Figure 10. Country Ranking of Imported Medical Device
- Provinces Analysis of Domestic Medical Device
The top five provinces are Beijing, Jiangsu, Guangdong, Shanghai, Shandong, which make up 67% of first and renew registration in 2015.

Figure 11. Distribution diagram of domestic medical device

Figure 12. Province ranking of domestic medical device
4.1 Domestic medical device of Class II
By 2015, there are 12284 medical device of class II approved by province FDA, include 5566 first registrations, 4981 renew registrations, and 1800 changes.
Figure 13. Scatter gram of registration form of Domestic Medical Device of Class II
Province Data:
Figure 14. Scatter gram of registration form of Domestic Medical Device of Class II

