1. Introduction
Product Name: Blood Glucose Test Strips (Supporting product: blood glucose tester)
Product Composition:
Generally, it consists of an electrode, a reagent, a protective cover film, and a PET substrate. The reagent is generally composed of an enzyme substance (glucose dehydrogenase, glucose oxidase), an enzyme protective agent, and a non-reactive component.
Working Principle:
1) Electrochemical method
pyrroloquinoline quinine
Electrochemical method uses the principle of detecting the current signal generated during the enzyme reaction to reflect the blood glucose level. The electrons generated by the reaction between the enzyme and glucose pass through the current counting device, read the quantity of electrons, and then convert it into a glucose concentration reading.
According to the enzyme used in the electrochemical blood glucose test strip, it is divided into two types: glucose oxidase (GOD) method and glucose dehydrogenase (GDH) method. GDH is also required to use different coenzymes in the reaction, namely pyrroloquinoline quinine glucose dehydrogenase (PQQ-GDH) and flavin adenine dinucleotide glucose dehydrogenase (FAD-GDH). And nicotinamide adenine dinucleotide glucose dehydrogenase (NAD-GDH).
2) Photochemical method
Photochemical method is to detect the color change of the test strip during the reaction to reflect the blood glucose level. The enzyme used in the blood glucose test strip is generally glucose oxidase (GOD), an intermediate (color-containing substance) produced by the reaction of the enzyme with glucose. After the reaction, the color of the test paper is changed, and the absorbance of the reflective surface of the test paper is detected by a detector, and the blood glucose concentration can be obtained according to Lambert-Beer's law.
Typical Structure:
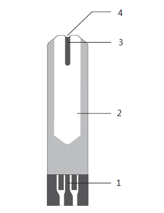
1. Electrode receiving area: Insert the area of the machine and accept the current generated after the reaction.
2. Handheld area: Take the part of the test strip.
3. Reaction zone: The area in which glucose in the blood reacts with chemical reagents.
4. Inhalation zone: Place blood drops close to this area and blood can be automatically inhaled into the reaction zone.
2. Classification
Classification Code (IVD Reagent): II-2 Class II Reagent for sugar detection
Product Description: It is used to detect the content of glucose in human samples, which is mainly used to reflect blood sugar levels.
3. Registration Unit
Divided into the same registration unit:
1. The composition is the same and can include different packaging specifications (difference in the number of test strips);
2. Different packaging specifications (difference in the number of test strips) and applicable to different types of instruments;
Divided into different registration units:
1. The reaction enzymes of blood glucose test strips are different;
2. Applicable objects (adults/babies);
The calibrator and quality control can be registered with the blood glucose test strip or applied for registration separately. The same registration unit can contain only calibrators or control products, as well as calibrators and controls.
4. Technical Requirements
Reference to the following standards:
GB/T 19634-2005 In Vitro Diagnostic Test System General Technical Conditions for Self-Test Blood Glucose Monitoring System
4.1 Appearance
The blood glucose test strip should be smooth and burr-free, and the positive sample loading area should be clean and free of stains, and the external markings should be clear.
4.2 Blood glucose test strip measurement repeatability
The precision of repeating measurement results of blood glucose test strips should meet the following requirements
Test range | Precision |
<5.5mmol/L(<100mg/dL) | SD<0.42 mmol/L(<7.7mg/dL) |
≥5.5mmol/L(≥100mg/dL) | CV<7.5% |
4.3 Accuracy of blood glucose meter and blood glucose test strip system:
95% of the deviation of the measurement results of the blood glucose meter and the supporting blood glucose test strip shall meet the requirements of the following table;
The recovery rate of glucose from blood glucose meter and blood glucose test strip is 80% to 120%.
Test range | Allowable deviation |
≤4.2mmol/L(≤75mg/dL) | No more than±0.83mmol/L(±15mg/dL) |
>4.2mmol/L(>75mg/dL) | No more than ±20% |
4.4 blood glucose test paper batch difference
The batch-to-batch difference between different batches of blood glucose test strips should be no more than 15%.
4.5 Control substances
95% of the control substance measurement results should be within the control range of the blood glucose test strip.
For the test method, see "GB/T 19634-2005 In vitro diagnostic test system General technical conditions for self-test blood glucose monitoring system ".
Table 1 Relevant Product Standards
Standard Number | Standard Name |
GB/T191-2008 | Packaging storage and transportation icon |
GB/T 19634-2005 | In vitro diagnostic test system General technical conditions for self-test blood glucose monitoring system |
YY(/T): Industry standard (Recommendation)
GB(/T): National standard (Recommendation)
5. Clinical Trials
Animal Test or Small Sample Test: not required
Clinical Trials: not required
6. Registration Cost and Duration
1) Registration Cost
Containing clinical evaluation cost
2) Technology Approval
60 working days
Another 60 working days if the 1-year supplement is required
3) Administrative Approval
20 working days
If you would like to get the detailed registration proposal, please contact us via md@cirs-group.com.

