Soft Contact Lens is an eye lens designed to be worn on the front surface of the eye and supported to maintain shape, it is managed as class III medical device under China National Medical Product Administration (Known as NMPA) regulations. Any Soft Contact Lens entering Chinese market are required to obtain registration certificate from NMPA.
Classification
Soft Contact Lens is classified as class III medical device, the MD code is 16-06-01 Contact Lens.
The Flowchart and timeframe of the Soft Contact Lens Registration Process 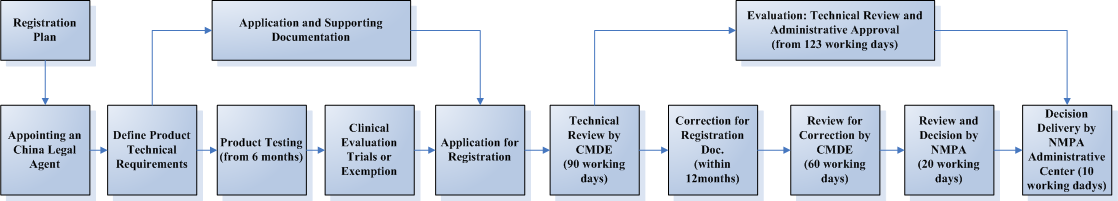
The registration process can be divided into 5 steps:
1. Appointing an China Legal Agent
2. Testing in a designated laboratory in China
3. Clinical evaluation via trial or exemption
4. Dossier preparation and submission
5. Technical evaluation and administrative approval
Clinical Trial Requirement
If the product are managed as Class II/III medical devices and not included in the Clinical Trial Exempted Catalog, Clinical trials will be required.
Soft Contact Lens is the Class III medical device but it is included in the 4th batch clinical trial exempted catalog (exposure draft), it will be exempted from clinical trial and only the clinical evaluating by comparing with the same variety product already approved in China.
The clinical trial exemption described on 4th batch clinical trial exempted medical devices catalog:
New Classification | Category | Product name | Product description |
16-06-01 | III | Soft Contact Lens | Day wear, single-focal design, correction of myopia or hyperopia. The product is of the same variety with the approved domestic registered product, and the product formula is mature. Except for new structure design, new mechanism of action and combination of medicine and apparatus. |
If the Soft Contact Lens meets the requirements of clinical trial exemption, it can be exempted from clinical trial during the registration in China.

